Hallmarks of Aging: Difference between revisions
| Line 169: | Line 169: | ||
!Experimentally increasing it accelerates aging | !Experimentally increasing it accelerates aging | ||
!Experimentally amending it slows the normal aging process and increases healthy lifespan. | !Experimentally amending it slows the normal aging process and increases healthy lifespan. | ||
!Associated human diseases | |||
|- | |- | ||
| style="text-align:center; background-color:hsla(180, 100%, 85%);" |[[File:DNA Structure+Key+Labelled.pn NoBB.png|frameless|76x76px]] | | style="text-align:center; background-color:hsla(180, 100%, 85%);" |[[File:DNA Structure+Key+Labelled.pn NoBB.png|frameless|76x76px]] | ||
| Line 176: | Line 177: | ||
|Deficient DNA repair causes premature aging{{pmid|19812404}} | |Deficient DNA repair causes premature aging{{pmid|19812404}} | ||
|Increased DNA repair facilitates greater longevity{{pmid|19812404}} | |Increased DNA repair facilitates greater longevity{{pmid|19812404}} | ||
| | |||
|- | |- | ||
| style="text-align:center; background-color:hsla(180, 100%, 85%);" |[[File:Telomeres transparent.png|frameless|88x88px]] | | style="text-align:center; background-color:hsla(180, 100%, 85%);" |[[File:Telomeres transparent.png|frameless|88x88px]] | ||
| style="background-color:hsla(180, 100%, 85%);" |'''Telomere attrition''' | | style="background-color:hsla(180, 100%, 85%);" |'''Telomere attrition''' | ||
| style="background-color:hsla(180, 100%, 85%);" | | | style="background-color:hsla(180, 100%, 85%);" |Telomere attrition is a hallmark characterized by the progressive shortening of telomeres, due to the inability of DNA polymerases to fully replicate the ends of linear chromosomes. This shortening contributes to genomic instability, cellular aging, and limited cellular lifespan. Telomeres are protected by shelterin complexes; however, their deterioration or the absence of telomerase leads to cellular senescence and diseases.{{pmid|17024208}}{{pmid|18680434}}{{pmid|22426077}}{{pmid|22965356}}{{pmid|18252230}}{{pmid|18669893}}{{pmid|21205863}}{{pmid|20569239}} | ||
| | |Telomere shortening is observed during normal aging in humans and mice{{pmid|17876321}}. | ||
| | |Telomerase deficiency and severe uncapping of telomeres lead to premature aging and diseases{{pmid|22965356}}{{pmid|18680434}}{{pmid|18252230}}{{pmid|18669893}}{{pmid|21205863}}. | ||
|Extension of telomeres through telomerase reactivation or other means can delay aging and extend lifespan without increasing cancer risk{{pmid|21113150}}{{pmid|22585399}}{{pmid|21426483}}{{pmid|23346961}}. | |||
| | | | ||
|- | |- | ||
| Line 187: | Line 190: | ||
| style="background-color:hsla(180, 100%, 85%);" |'''Epigenetic alterations''' | | style="background-color:hsla(180, 100%, 85%);" |'''Epigenetic alterations''' | ||
| style="background-color:hsla(180, 100%, 85%);" | | | style="background-color:hsla(180, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 194: | Line 198: | ||
| style="background-color:hsla(210, 100%, 85%);" |'''Loss of proteostasis''' | | style="background-color:hsla(210, 100%, 85%);" |'''Loss of proteostasis''' | ||
| style="background-color:hsla(210, 100%, 85%);" | | | style="background-color:hsla(210, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 200: | Line 205: | ||
| style="text-align:center; background-color:hsla(210, 100%, 85%);" |[[File:Macro-micro-autophagy.gif|frameless|101x101px]] | | style="text-align:center; background-color:hsla(210, 100%, 85%);" |[[File:Macro-micro-autophagy.gif|frameless|101x101px]] | ||
| style="background-color:hsla(210, 100%, 85%);" |'''Disabled autophagy''' | | style="background-color:hsla(210, 100%, 85%);" |'''Disabled autophagy''' | ||
| style="background-color:hsla(210, 100%, 85%);" | | | style="background-color:hsla(210, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 207: | Line 213: | ||
| style="text-align:center; background-color:hsla(0, 100%, 85%);" |[[File:Aiga restaurant knife-fork crossed.png|frameless|75x75px]] | | style="text-align:center; background-color:hsla(0, 100%, 85%);" |[[File:Aiga restaurant knife-fork crossed.png|frameless|75x75px]] | ||
| style="background-color:hsla(0, 100%, 85%);" |'''Deregulated nutrient sensing''' | | style="background-color:hsla(0, 100%, 85%);" |'''Deregulated nutrient sensing''' | ||
| style="background-color:hsla(0, 100%, 85%);" | | | style="background-color:hsla(0, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 215: | Line 222: | ||
| style="background-color:hsla(0, 100%, 85%);" |'''Mitochondrial dysfunction''' | | style="background-color:hsla(0, 100%, 85%);" |'''Mitochondrial dysfunction''' | ||
| style="background-color:hsla(0, 100%, 85%);" | | | style="background-color:hsla(0, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 222: | Line 230: | ||
| style="background-color:hsla(30, 100%, 85%);" |[[Senescent Cells|'''Cellular senescence''']] | | style="background-color:hsla(30, 100%, 85%);" |[[Senescent Cells|'''Cellular senescence''']] | ||
| style="background-color:hsla(30, 100%, 85%);" | | | style="background-color:hsla(30, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 229: | Line 238: | ||
| style="background-color:hsla(30, 100%, 85%);" |'''Stem cell exhaustion''' | | style="background-color:hsla(30, 100%, 85%);" |'''Stem cell exhaustion''' | ||
| style="background-color:hsla(30, 100%, 85%);" | | | style="background-color:hsla(30, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 237: | Line 247: | ||
'''(Microbiome disturbance)''' | '''(Microbiome disturbance)''' | ||
| style="background-color:hsla(30, 100%, 85%);" | | | style="background-color:hsla(30, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 245: | Line 256: | ||
'''(Inflammaging)''' | '''(Inflammaging)''' | ||
| style="background-color:hsla(30, 100%, 85%);" | | | style="background-color:hsla(30, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| Line 252: | Line 264: | ||
| style="background-color:hsla(30, 100%, 85%);" |'''Altered intercellular communication''' | | style="background-color:hsla(30, 100%, 85%);" |'''Altered intercellular communication''' | ||
| style="background-color:hsla(30, 100%, 85%);" | | | style="background-color:hsla(30, 100%, 85%);" | | ||
| | |||
| | | | ||
| | | | ||
| | | | ||
|} | |} | ||
==History== | ==History== | ||
Revision as of 20:10, 3 January 2024
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. The hallmarks of aging are the types of biochemical changes that occur in all organisms that experience biological aging and lead to a progressive loss of physiological integrity, impaired function and, eventually, death. They were first listed in a landmark paper in 2013[1] to conceptualize the essence of biological aging and its underlying mechanisms.
Criteria
Each hallmark was chosen to try to fulfill the following criteria:[1]
- manifests during normal aging;
- experimentally increasing it accelerates aging;
- experimentally amending it slows the normal aging process and increases healthy lifespan.
These conditions are met to different extents by each of these hallmarks. The last criterion is not present in many of the hallmarks, as science has not yet found feasible ways to amend these problems in living organisms.
Overview
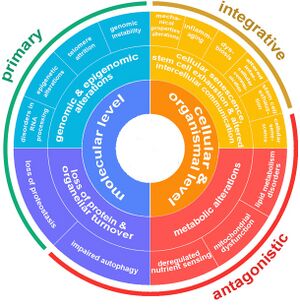
Aging is a complex process characterized by a gradual decline in physiological function. The hallmarks of aging are classified into three categories, each describing different aspects of the aging process:
- Primary Hallmarks: These are considered the main causes of cellular damage leading to aging. They are the initiating factors that, over time, drive the functional decline seen in aging cells and tissues.
- Antagonistic Hallmarks: These hallmarks are the response to the damage caused by the primary hallmarks. Initially, they may be compensatory or protective, but when chronic or excessive, they become deleterious, contributing to the aging process.
- Integrative Hallmarks: These hallmarks are the culprits of the phenotype of aging. They result from a combination of the primary and antagonistic hallmarks and are ultimately responsible for the functional decline in tissues and organs seen in aging.
The Nine Hallmarks of Aging (2013)
The nine hallmarks of aging were originally conceptualized by López-Otín and colleagues in 2013[1]. Since that it has served as a foundational paradigm for aging research for a decade until it has been revised in 2022. The original 9 landmarks were defined as follows:
| # | Hallmark | |
|---|---|---|
| 1 | Genomic Instability | Primary Hallmarks
(causes damage) |
| 2 | Telomere Attrition | |
| 3 | Epigenetic Alterations | |
| 4 | Loss of Proteostasis | |
| 5 | Deregulated Nutrient Sensing | Antagonistic Hallmarks
(responses to damage) |
| 6 | Mitochondrial Dysfunction | |
| 7 | Cellular Senescence | |
| 8 | Stem Cell Exhaustion | Integrative Hallmarks
(culprits of the phenotype) |
| 9 | Altered Intercellular Communication |
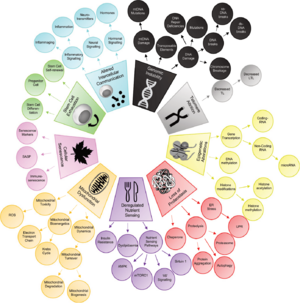
Five New Hallmarks of Aging (2022)
While these nine hallmarks have significantly advanced our understanding of aging and its relation to age-related diseases, recent critiques and evolving scientific evidence have prompted the scientific community to reconsider and expand this framework.[4] To address this, a symposium titled “New Hallmarks of Ageing” was held in Copenhagen on March 2022, where leading experts gathered to discuss potential additions and recontextualizations of these aging hallmarks. The symposium highlighted the critical need for an expanded, inclusive paradigm that encompasses newly identified processes contributing to aging. The discussions suggested the integration of five additional hallmarks:
- compromised autophagy
- dysregulation in RNA splicing
- inflammation
- loss of cytoskeleton integrity
- disturbance of the microbiome (dysbiosis)
These potential new hallmarks, along with the original nine, underscore a more comprehensive understanding of the aging process, acknowledging its multifaceted nature and its profound implications for human health and longevity.[5]
The Twelve Hallmarks of Aging (2023)
| Level | Hallmark | Description | Proposed Year |
Category | |
|---|---|---|---|---|---|
| Molecular Level |

|
Genomic instability | Accumulation of DNA damage over time leading to cellular dysfunction. | 2013 | Primary Hallmarks (causes damage) |
|
Telomere attrition | Reduction in the length of telomeres leading to cellular aging. | 2013 | ||

|
Epigenetic alterations | Changes in DNA methylation and histone modification affecting gene expression. | 2013 | ||
|
Loss of proteostasis | Disruption in protein folding and stability leading to cell damage. | 2013 | ||

|
Disabled autophagy | Impaired cellular maintenance through the consumption of own components. | 2021[6] | Antagonistic Hallmarks (responses to damage) | |
| Cellular & Organismal Level |

|
Deregulated nutrient sensing | Alterations in nutrient sensing pathways affecting metabolism and aging. | 2013 | |
|
Mitochondrial dysfunction | Decrease in mitochondrial efficiency and increase in oxidative stress. | 2013 | ||

|
Cellular senescence | Accumulation of non-dividing, dysfunctional cells secreting harmful factors. | 2013 | ||
|
Stem cell exhaustion | Decline in the regenerative capacity of stem cells affecting tissue repair. | 2013 | Integrative Hallmarks (culprits of the phenotype) | |
|
Dysbiosis
(Microbiome disturbance) |
Changes in gut microbiome affecting health and aging. | |||

|
Chronic inflammation
(Inflammaging) |
Systemic inflammation contributing to aging and related diseases. | 2023[7] | ||

|
Altered intercellular communication | Changes in cellular communication leading to inflammation and tissue dysfunction. | 2013 | ||
Potential Hallmarks of Aging

|
Altered mechanical properties | Changes in cellular and extracellular structure affecting tissue function. | |

|
Splicing dysregulation
(Dysregulation in RNA splicing) |
Impaired RNA construction from DNA, leading to cellular dysfunction. | 2019[8] |
The Hallmarks in Detail
| Hallmark | Background | Manifests during normal aging | Experimentally increasing it accelerates aging | Experimentally amending it slows the normal aging process and increases healthy lifespan. | Associated human diseases | |
|---|---|---|---|---|---|---|

|
Genomic instability | Damange in the DNA are formed mainly through oxidative stress and environmental factors.[9] A number of molecular processes work continuously to repair this damage.[10] | DNA damage accumulates over time[11] | Deficient DNA repair causes premature aging[12] | Increased DNA repair facilitates greater longevity[12] | |

|
Telomere attrition | Telomere attrition is a hallmark characterized by the progressive shortening of telomeres, due to the inability of DNA polymerases to fully replicate the ends of linear chromosomes. This shortening contributes to genomic instability, cellular aging, and limited cellular lifespan. Telomeres are protected by shelterin complexes; however, their deterioration or the absence of telomerase leads to cellular senescence and diseases.[13][14][15][16][17][18][19][20] | Telomere shortening is observed during normal aging in humans and mice[21]. | Telomerase deficiency and severe uncapping of telomeres lead to premature aging and diseases[16][14][17][18][19]. | Extension of telomeres through telomerase reactivation or other means can delay aging and extend lifespan without increasing cancer risk[22][23][24][25]. | |

|
Epigenetic alterations | |||||

|
Loss of proteostasis | |||||

|
Disabled autophagy | |||||

|
Deregulated nutrient sensing | |||||

|
Mitochondrial dysfunction | |||||

|
Cellular senescence | |||||

|
Stem cell exhaustion | |||||

|
Dysbiosis
(Microbiome disturbance) |
|||||

|
Chronic inflammation
(Inflammaging) |
|||||

|
Altered intercellular communication | |||||
History
- 2013 The scientific journal Cell published the article "The Hallmarks of Aging", that was translated to several languages and determined the directions of many studies.[1]
- 2022 It was proposed to expand the list of the nine hallmarks of aging with five more.[5][26]
- 2023 In a paywalled review, the authors of a heavily cited paper on the hallmarks of aging update the set of proposed hallmarks after a decade.[27] A review with overlapping authors merge or link various hallmarks of cancer with those of aging.[28]
Todo
- 2013, The hallmarks of aging [1]
- 2023, Chronic inflammation and the hallmarks of aging [7]
- 2023, Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement [2]
- 2022, Biological mechanisms of aging predict age-related disease co-occurrence in patients [3]
- https://doi.org/10.1038/s41587-023-02024-y
References
- ↑ 1.0 1.1 1.2 1.3 1.4 López-Otín C et al.: The hallmarks of aging. Cell 2013. (PMID 23746838) [PubMed] [DOI] [Full text] Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. This deterioration is the primary risk factor for major human pathologies, including cancer, diabetes, cardiovascular disorders, and neurodegenerative diseases. Aging research has experienced an unprecedented advance over recent years, particularly with the discovery that the rate of aging is controlled, at least to some extent, by genetic pathways and biochemical processes conserved in evolution. This Review enumerates nine tentative hallmarks that represent common denominators of aging in different organisms, with special emphasis on mammalian aging. These hallmarks are: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. A major challenge is to dissect the interconnectedness between the candidate hallmarks and their relative contributions to aging, with the final goal of identifying pharmaceutical targets to improve human health during aging, with minimal side effects.
- ↑ 2.0 2.1 Tenchov R et al.: Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem Neurosci 2023. (PMID 38095562) [PubMed] [DOI] Aging is a dynamic, time-dependent process that is characterized by a gradual accumulation of cell damage. Continual functional decline in the intrinsic ability of living organisms to accurately regulate homeostasis leads to increased susceptibility and vulnerability to diseases. Many efforts have been put forth to understand and prevent the effects of aging. Thus, the major cellular and molecular hallmarks of aging have been identified, and their relationships to age-related diseases and malfunctions have been explored. Here, we use data from the CAS Content Collection to analyze the publication landscape of recent aging-related research. We review the advances in knowledge and delineate trends in research advancements on aging factors and attributes across time and geography. We also review the current concepts related to the major aging hallmarks on the molecular, cellular, and organismic level, age-associated diseases, with attention to brain aging and brain health, as well as the major biochemical processes associated with aging. Major age-related diseases have been outlined, and their correlations with the major aging features and attributes are explored. We hope this review will be helpful for apprehending the current knowledge in the field of aging mechanisms and progression, in an effort to further solve the remaining challenges and fulfill its potential.
- ↑ 3.0 3.1 Fraser HC et al.: Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell 2022. (PMID 35259281) [PubMed] [DOI] [Full text] Genetic, environmental, and pharmacological interventions into the aging process can confer resistance to multiple age-related diseases in laboratory animals, including rhesus monkeys. These findings imply that individual mechanisms of aging might contribute to the co-occurrence of age-related diseases in humans and could be targeted to prevent these conditions simultaneously. To address this question, we text mined 917,645 literature abstracts followed by manual curation and found strong, non-random associations between age-related diseases and aging mechanisms in humans, confirmed by gene set enrichment analysis of GWAS data. Integration of these associations with clinical data from 3.01 million patients showed that age-related diseases associated with each of five aging mechanisms were more likely than chance to be present together in patients. Genetic evidence revealed that innate and adaptive immunity, the intrinsic apoptotic signaling pathway and activity of the ERK1/2 pathway were associated with multiple aging mechanisms and diverse age-related diseases. Mechanisms of aging hence contribute both together and individually to age-related disease co-occurrence in humans and could potentially be targeted accordingly to prevent multimorbidity.
- ↑ Gems D & de Magalhães JP: The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Res Rev 2021. (PMID 34271186) [PubMed] [DOI] [Full text] With the goal of representing common denominators of aging in different organisms López-Otín et al. in 2013 described nine hallmarks of aging. Since then, this representation has become a major reference point for the biogerontology field. The template for the hallmarks of aging account originated from landmark papers by Hanahan and Weinberg (2000, 2011) defining first six and later ten hallmarks of cancer. Here we assess the strengths and weaknesses of the hallmarks of aging account. As a checklist of diverse major foci of current aging research, it has provided a useful shared overview for biogerontology during a time of transition in the field. It also seems useful in applied biogerontology, to identify interventions (e.g. drugs) that impact multiple symptomatic features of aging. However, while the hallmarks of cancer provide a paradigmatic account of the causes of cancer with profound explanatory power, the hallmarks of aging do not. A worry is that as a non-paradigm the hallmarks of aging have obscured the urgent need to define a genuine paradigm, one that can provide a useful basis for understanding the mechanistic causes of the diverse aging pathologies. We argue that biogerontology must look and move beyond the hallmarks to understand the process of aging.
- ↑ 5.0 5.1 Schmauck-Medina T et al.: New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany NY) 2022. (PMID 36040386) [PubMed] [DOI] [Full text] Genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, deregulated nutrient-sensing, cellular senescence, stem cell exhaustion, and altered intercellular communication were the original nine hallmarks of ageing proposed by López-Otín and colleagues in 2013. The proposal of these hallmarks of ageing has been instrumental in guiding and pushing forward research on the biology of ageing. In the nearly past 10 years, our in-depth exploration on ageing research has enabled us to formulate new hallmarks of ageing which are compromised autophagy, microbiome disturbance, altered mechanical properties, splicing dysregulation, and inflammation, among other emerging ones. Amalgamation of the 'old' and 'new' hallmarks of ageing may provide a more comprehensive explanation of ageing and age-related diseases, shedding light on interventional and therapeutic studies to achieve healthy, happy, and productive lives in the elderly.
- ↑ Kaushik S et al.: Autophagy and the hallmarks of aging. Ageing Res Rev 2021. (PMID 34563704) [PubMed] [DOI] [Full text] Autophagy, an essential cellular process that mediates degradation of proteins and organelles in lysosomes, has been tightly linked to cellular quality control for its role as part of the proteostasis network. The current interest in identifying the cellular and molecular determinants of aging, has highlighted the important contribution of malfunctioning of autophagy with age to the loss of proteostasis that characterizes all old organisms. However, the diversity of cellular functions of the different types of autophagy and the often reciprocal interactions of autophagy with other determinants of aging, is placing autophagy at the center of the aging process. In this work, we summarize evidence for the contribution of autophagy to health- and lifespan and provide examples of the bidirectional interplay between autophagic pathways and several of the so-called hallmarks of aging. This central role of autophagy in aging, and the dependence on autophagy of many geroprotective interventions, has motivated a search for direct modulators of autophagy that could be used to slow aging and extend healthspan. Here, we review some of those ongoing therapeutic efforts and comment on the potential of targeting autophagy in aging.
- ↑ 7.0 7.1 Baechle JJ et al.: Chronic inflammation and the hallmarks of aging. Mol Metab 2023. (PMID 37329949) [PubMed] [DOI] [Full text] BACKGROUND: Recently, the hallmarks of aging were updated to include dysbiosis, disabled macroautophagy, and chronic inflammation. In particular, the low-grade chronic inflammation during aging, without overt infection, is defined as "inflammaging," which is associated with increased morbidity and mortality in the aging population. Emerging evidence suggests a bidirectional and cyclical relationship between chronic inflammation and the development of age-related conditions, such as cardiovascular diseases, neurodegeneration, cancer, and frailty. How the crosstalk between chronic inflammation and other hallmarks of aging underlies biological mechanisms of aging and age-related disease is thus of particular interest to the current geroscience research. SCOPE OF REVIEW: This review integrates the cellular and molecular mechanisms of age-associated chronic inflammation with the other eleven hallmarks of aging. Extra discussion is dedicated to the hallmark of "altered nutrient sensing," given the scope of Molecular Metabolism. The deregulation of hallmark processes during aging disrupts the delicate balance between pro-inflammatory and anti-inflammatory signaling, leading to a persistent inflammatory state. The resultant chronic inflammation, in turn, further aggravates the dysfunction of each hallmark, thereby driving the progression of aging and age-related diseases. MAIN CONCLUSIONS: The crosstalk between chronic inflammation and other hallmarks of aging results in a vicious cycle that exacerbates the decline in cellular functions and promotes aging. Understanding this complex interplay will provide new insights into the mechanisms of aging and the development of potential anti-aging interventions. Given their interconnectedness and ability to accentuate the primary elements of aging, drivers of chronic inflammation may be an ideal target with high translational potential to address the pathological conditions associated with aging.
- ↑ Bhadra M et al.: Alternative splicing in aging and longevity. Hum Genet 2020. (PMID 31834493) [PubMed] [DOI] [Full text] Alternative pre-mRNA splicing increases the complexity of the proteome that can be generated from the available genomic coding sequences. Dysregulation of the splicing process has been implicated in a vast repertoire of diseases. However, splicing has recently been linked to both the aging process itself and pro-longevity interventions. This review focuses on recent research towards defining RNA splicing as a new hallmark of aging. We highlight dysfunctional alternative splicing events that contribute to the aging phenotype across multiple species, along with recent efforts toward deciphering mechanistic roles for RNA splicing in the regulation of aging and longevity. Further, we discuss recent research demonstrating a direct requirement for specific splicing factors in pro-longevity interventions, and specifically how nutrient signaling pathways interface to splicing factor regulation and downstream splicing targets. Finally, we review the emerging potential of using splicing profiles as a predictor of biological age and life expectancy. Understanding the role of RNA splicing components and downstream targets altered in aging may provide opportunities to develop therapeutics and ultimately extend healthy lifespan in humans.
- ↑ De Bont R & van Larebeke N: Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 2004. (PMID 15123782) [PubMed] [DOI] DNA damage plays a major role in mutagenesis, carcinogenesis and ageing. The vast majority of mutations in human tissues are certainly of endogenous origin. A thorough knowledge of the types and prevalence of endogenous DNA damage is thus essential for an understanding of the interactions of endogenous processes with exogenous agents and the influence of damage of endogenous origin on the induction of cancer and other diseases. In particular, this seems important in risk evaluation concerning exogenous agents that also occur endogenously or that, although chemically different from endogenous ones, generate the same DNA adducts. This knowledge may also be crucial to the development of rational chemopreventive strategies. A list of endogenous DNA-damaging agents, processes and DNA adduct levels is presented. For the sake of comparison, DNA adduct levels are expressed in a standardized way, including the number of adducts per 10(6) nt. This list comprises numerous reactive oxygen species and products generated as a consequence (e.g. lipid peroxides), endogenous reactive chemicals (e.g. aldehydes and S-adenosylmethionine), and chemical DNA instability (e.g. depurination). The respective roles of endogenous versus exogenous DNA damage in carcinogenesis are discussed.
- ↑ de Duve C: The onset of selection. Nature 2005. (PMID 15703726) [PubMed] [DOI]
- ↑ Vijg J & Suh Y: Genome instability and aging. Annu Rev Physiol 2013. (PMID 23398157) [PubMed] [DOI] Genome instability has long been implicated as the main causal factor in aging. Somatic cells are continuously exposed to various sources of DNA damage, from reactive oxygen species to UV radiation to environmental mutagens. To cope with the tens of thousands of chemical lesions introduced into the genome of a typical cell each day, a complex network of genome maintenance systems acts to remove damage and restore the correct base pair sequence. Occasionally, however, repair is erroneous, and such errors, as well as the occasional failure to correctly replicate the genome during cell division, are the basis for mutations and epimutations. There is now ample evidence that mutations accumulate in various organs and tissues of higher animals, including humans, mice, and flies. What is not known, however, is whether the frequency of these random changes is sufficient to cause the phenotypic effects generally associated with aging. The exception is cancer, an age-related disease caused by the accumulation of mutations and epimutations. Here, we first review current concepts regarding the relationship between DNA damage, repair, and mutation, as well as the data regarding genome alterations as a function of age. We then describe a model for how randomly induced DNA sequence and epigenomic variants in the somatic genomes of animals can result in functional decline and disease in old age. Finally, we discuss the genetics of genome instability in relation to longevity to address the importance of alterations in the somatic genome as a causal factor in aging and to underscore the opportunities provided by genetic approaches to develop interventions that attenuate genome instability, reduce disease risk, and increase life span.
- ↑ 12.0 12.1 Hoeijmakers JH: DNA damage, aging, and cancer. N Engl J Med 2009. (PMID 19812404) [PubMed] [DOI]
- ↑ Blackburn EH et al.: Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 2006. (PMID 17024208) [PubMed] [DOI]
- ↑ 14.0 14.1 Palm W & de Lange T: How shelterin protects mammalian telomeres. Annu Rev Genet 2008. (PMID 18680434) [PubMed] [DOI] The genomes of prokaryotes and eukaryotic organelles are usually circular as are most plasmids and viral genomes. In contrast, the nuclear genomes of eukaryotes are organized on linear chromosomes, which require mechanisms to protect and replicate DNA ends. Eukaryotes navigate these problems with the advent of telomeres, protective nucleoprotein complexes at the ends of linear chromosomes, and telomerase, the enzyme that maintains the DNA in these structures. Mammalian telomeres contain a specific protein complex, shelterin, that functions to protect chromosome ends from all aspects of the DNA damage response and regulates telomere maintenance by telomerase. Recent experiments, discussed here, have revealed how shelterin represses the ATM and ATR kinase signaling pathways and hides chromosome ends from nonhomologous end joining and homology-directed repair.
- ↑ Fumagalli M et al.: Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 2012. (PMID 22426077) [PubMed] [DOI] [Full text] The DNA-damage response (DDR) arrests cell-cycle progression until damage is removed. DNA-damage-induced cellular senescence is associated with persistent DDR. The molecular bases that distinguish transient from persistent DDR are unknown. Here we show that a large fraction of exogenously induced persistent DDR markers is associated with telomeric DNA in cultured cells and mammalian tissues. In yeast, a chromosomal DNA double-strand break next to a telomeric sequence resists repair and impairs DNA ligase 4 recruitment. In mammalian cells, ectopic localization of telomeric factor TRF2 next to a double-strand break induces persistent DNA damage and DDR. Linear, but not circular, telomeric DNA or scrambled DNA induces a prolonged checkpoint in normal cells. In terminally differentiated tissues of old primates, DDR markers accumulate at telomeres that are not critically short. We propose that linear genomes are not uniformly reparable and that telomeric DNA tracts, if damaged, are irreparable and trigger persistent DDR and cellular senescence.
- ↑ 16.0 16.1 Armanios M & Blackburn EH: The telomere syndromes. Nat Rev Genet 2012. (PMID 22965356) [PubMed] [DOI] [Full text] There has been mounting evidence of a causal role for telomere dysfunction in a number of degenerative disorders. Their manifestations encompass common disease states such as idiopathic pulmonary fibrosis and bone marrow failure. Although these disorders seem to be clinically diverse, collectively they comprise a single syndrome spectrum defined by the short telomere defect. Here we review the manifestations and unique genetics of telomere syndromes. We also discuss their underlying molecular mechanisms and significance for understanding common age-related disease processes.
- ↑ 17.0 17.1 Savage SA et al.: TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet 2008. (PMID 18252230) [PubMed] [DOI] [Full text] Patients with dyskeratosis congenita (DC), a heterogeneous inherited bone marrow failure syndrome, have abnormalities in telomere biology, including very short telomeres and germline mutations in DKC1, TERC, TERT, or NOP10, but approximately 60% of DC patients lack an identifiable mutation. With the very short telomere phenotype and a highly penetrant, rare disease model, a linkage scan was performed on a family with autosomal-dominant DC and no mutations in DKCI, TERC, or TERT. Evidence favoring linkage was found at 2p24 and 14q11.2, and this led to the identification of TINF2 (14q11.2) mutations, K280E, in the proband and her five affected relatives and TINF2 R282H in three additional unrelated DC probands, including one with Revesz syndrome; a fifth DC proband had a R282S mutation. TINF2 mutations were not present in unaffected relatives, DC probands with mutations in DKC1, TERC, or TERT or 298 control subjects. We demonstrate that a fifth gene, TINF2, is mutated in classical DC and, for the first time, in Revesz syndrome. This represents the first shelterin complex mutation linked to human disease and confirms the role of very short telomeres as a diagnostic test for DC.
- ↑ 18.0 18.1 Walne AJ et al.: TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood 2008. (PMID 18669893) [PubMed] [DOI] [Full text] Dyskeratosis congenita (DC) is a multisystem bone marrow failure syndrome characterized by a triad of mucocutaneous abnormalities and a predisposition to cancer. The genetic basis of DC remains unknown in more than 60% of patients. Mutations have been identified in components of the telomerase complex (dyskerin, TERC, TERT, NOP10, and NHP2), and recently in one component of the shelterin complex TIN2 (gene TINF2). To establish the role of TINF2 mutations, we screened DNA from 175 uncharacterised patients with DC as well as 244 patients with other bone marrow failure disorders. Heterozygous coding mutations were found in 33 of 175 previously uncharacterized DC index patients and 3 of 244 other patients. A total of 21 of the mutations affected amino acid 282, changing arginine to histidine (n = 14) or cysteine (n = 7). A total of 32 of 33 patients with DC with TINF2 mutations have severe disease, with most developing aplastic anaemia by the age of 10 years. Telomere lengths in patients with TINF2 mutations were the shortest compared with other DC subtypes, but TERC levels were normal. In this large series, TINF2 mutations account for approximately 11% of all DC, but they do not play a significant role in patients with related disorders. This study emphasises the role of defective telomere maintenance on human disease.
- ↑ 19.0 19.1 Zhong F et al.: Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev 2011. (PMID 21205863) [PubMed] [DOI] [Full text] Dyskeratosis congenita (DC) is a genetic disorder of defective tissue maintenance and cancer predisposition caused by short telomeres and impaired stem cell function. Telomerase mutations are thought to precipitate DC by reducing either the catalytic activity or the overall levels of the telomerase complex. However, the underlying genetic mutations and the mechanisms of telomere shortening remain unknown for as many as 50% of DC patients, who lack mutations in genes controlling telomere homeostasis. Here, we show that disruption of telomerase trafficking accounts for unknown cases of DC. We identify DC patients with missense mutations in TCAB1, a telomerase holoenzyme protein that facilitates trafficking of telomerase to Cajal bodies. Compound heterozygous mutations in TCAB1 disrupt telomerase localization to Cajal bodies, resulting in misdirection of telomerase RNA to nucleoli, which prevents telomerase from elongating telomeres. Our findings establish telomerase mislocalization as a novel cause of DC, and suggest that telomerase trafficking defects may contribute more broadly to the pathogenesis of telomere-related disease.
- ↑ Martínez P & Blasco MA: Role of shelterin in cancer and aging. Aging Cell 2010. (PMID 20569239) [PubMed] [DOI] Mammalian telomeres are formed by tandem repeats of the TTAGGG sequence bound by a specialized six-protein complex known as shelterin, which has fundamental roles in the regulation of telomere length and telomere capping. In the past, the study of mice genetically modified for telomerase components has been instrumental to demonstrate the role of telomere length in cancer and aging. Recent studies using genetically modified mice for shelterin proteins have highlighted an equally important role of telomere-bound proteins in cancer and aging, even in the presence of proficient telomerase activity and normal telomere length. In this review, we will focus on recent findings, suggesting a role of shelterin components in cancer and aging.
- ↑ Blasco MA: Telomere length, stem cells and aging. Nat Chem Biol 2007. (PMID 17876321) [PubMed] [DOI] Telomere shortening occurs concomitant with organismal aging, and it is accelerated in the context of human diseases associated with mutations in telomerase, such as some cases of dyskeratosis congenita, idiopathic pulmonary fibrosis and aplastic anemia. People with these diseases, as well as Terc-deficient mice, show decreased lifespan coincidental with a premature loss of tissue renewal, which suggests that telomerase is rate-limiting for tissue homeostasis and organismal survival. These findings have gained special relevance as they suggest that telomerase activity and telomere length can directly affect the ability of stem cells to regenerate tissues. If this is true, stem cell dysfunction provoked by telomere shortening may be one of the mechanisms responsible for organismal aging in both humans and mice. Here, we will review the current evidence linking telomere shortening to aging and stem cell dysfunction.
- ↑ Jaskelioff M et al.: Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011. (PMID 21113150) [PubMed] [DOI] [Full text] An ageing world population has fuelled interest in regenerative remedies that may stem declining organ function and maintain fitness. Unanswered is whether elimination of intrinsic instigators driving age-associated degeneration can reverse, as opposed to simply arrest, various afflictions of the aged. Such instigators include progressively damaged genomes. Telomerase-deficient mice have served as a model system to study the adverse cellular and organismal consequences of wide-spread endogenous DNA damage signalling activation in vivo. Telomere loss and uncapping provokes progressive tissue atrophy, stem cell depletion, organ system failure and impaired tissue injury responses. Here, we sought to determine whether entrenched multi-system degeneration in adult mice with severe telomere dysfunction can be halted or possibly reversed by reactivation of endogenous telomerase activity. To this end, we engineered a knock-in allele encoding a 4-hydroxytamoxifen (4-OHT)-inducible telomerase reverse transcriptase-oestrogen receptor (TERT-ER) under transcriptional control of the endogenous TERT promoter. Homozygous TERT-ER mice have short dysfunctional telomeres and sustain increased DNA damage signalling and classical degenerative phenotypes upon successive generational matings and advancing age. Telomerase reactivation in such late generation TERT-ER mice extends telomeres, reduces DNA damage signalling and associated cellular checkpoint responses, allows resumption of proliferation in quiescent cultures, and eliminates degenerative phenotypes across multiple organs including testes, spleens and intestines. Notably, somatic telomerase reactivation reversed neurodegeneration with restoration of proliferating Sox2(+) neural progenitors, Dcx(+) newborn neurons, and Olig2(+) oligodendrocyte populations. Consistent with the integral role of subventricular zone neural progenitors in generation and maintenance of olfactory bulb interneurons, this wave of telomerase-dependent neurogenesis resulted in alleviation of hyposmia and recovery of innate olfactory avoidance responses. Accumulating evidence implicating telomere damage as a driver of age-associated organ decline and disease risk and the marked reversal of systemic degenerative phenotypes in adult mice observed here support the development of regenerative strategies designed to restore telomere integrity.
- ↑ Bernardes de Jesus B et al.: Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 2012. (PMID 22585399) [PubMed] [DOI] [Full text] A major goal in aging research is to improve health during aging. In the case of mice, genetic manipulations that shorten or lengthen telomeres result, respectively, in decreased or increased longevity. Based on this, we have tested the effects of a telomerase gene therapy in adult (1 year of age) and old (2 years of age) mice. Treatment of 1- and 2-year old mice with an adeno associated virus (AAV) of wide tropism expressing mouse TERT had remarkable beneficial effects on health and fitness, including insulin sensitivity, osteoporosis, neuromuscular coordination and several molecular biomarkers of aging. Importantly, telomerase-treated mice did not develop more cancer than their control littermates, suggesting that the known tumorigenic activity of telomerase is severely decreased when expressed in adult or old organisms using AAV vectors. Finally, telomerase-treated mice, both at 1-year and at 2-year of age, had an increase in median lifespan of 24 and 13%, respectively. These beneficial effects were not observed with a catalytically inactive TERT, demonstrating that they require telomerase activity. Together, these results constitute a proof-of-principle of a role of TERT in delaying physiological aging and extending longevity in normal mice through a telomerase-based treatment, and demonstrate the feasibility of anti-aging gene therapy.
- ↑ Bernardes de Jesus B et al.: The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 2011. (PMID 21426483) [PubMed] [DOI] [Full text] Here, we show that a small-molecule activator of telomerase (TA-65) purified from the root of Astragalus membranaceus is capable of increasing average telomere length and decreasing the percentage of critically short telomeres and of DNA damage in haploinsufficient mouse embryonic fibroblasts (MEFs) that harbor critically short telomeres and a single copy of the telomerase RNA Terc gene (G3 Terc(+/-) MEFs). Importantly, TA-65 does not cause telomere elongation or rescue DNA damage in similarly treated telomerase-deficient G3 Terc(-/-) littermate MEFs. These results indicate that TA-65 treatment results in telomerase-dependent elongation of short telomeres and rescue of associated DNA damage, thus demonstrating that TA-65 mechanism of action is through the telomerase pathway. In addition, we demonstrate that TA-65 is capable of increasing mouse telomerase reverse transcriptase levels in some mouse tissues and elongating critically short telomeres when supplemented as part of a standard diet in mice. Finally, TA-65 dietary supplementation in female mice leads to an improvement of certain health-span indicators including glucose tolerance, osteoporosis and skin fitness, without significantly increasing global cancer incidence.
- ↑ Boonekamp JJ et al.: Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 2013. (PMID 23346961) [PubMed] [DOI] Biomarkers of aging are essential to predict mortality and aging-related diseases. Paradoxically, age itself imposes a limitation on the use of known biomarkers of aging because their associations with mortality generally diminish with age. How this pattern arises is, however, not understood. With meta-analysis we show that human leucocyte telomere length (TL) predicts mortality, and that this mortality association diminishes with age, as found for other biomarkers of aging. Subsequently, we demonstrate with simulation models that this observation cannot be reconciled with the popular hypothesis that TL is proportional to biological age. Using the reliability theory of aging, we instead propose that TL is a biomarker of somatic redundancy, the body's capacity to absorb damage, which fits the observed pattern well. We discuss to what extent diminishing redundancy with age may also explain the observed diminishing mortality modulation with age of other biomarkers of aging. Considering diminishing somatic redundancy as the causal agent of aging may critically advance our understanding of the aging process, and improve predictions of life expectancy and vulnerability to aging-related diseases.
- ↑ Researchers Propose Five New Hallmarks of Aging, https://www.lifespan.io/news/researchers-propose-five-new-hallmarks-of-aging/
- ↑ López-Otín C et al.: Hallmarks of aging: An expanding universe. Cell 2023. (PMID 36599349) [PubMed] [DOI] Aging is driven by hallmarks fulfilling the following three premises: (1) their age-associated manifestation, (2) the acceleration of aging by experimentally accentuating them, and (3) the opportunity to decelerate, stop, or reverse aging by therapeutic interventions on them. We propose the following twelve hallmarks of aging: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis. These hallmarks are interconnected among each other, as well as to the recently proposed hallmarks of health, which include organizational features of spatial compartmentalization, maintenance of homeostasis, and adequate responses to stress.
- ↑ López-Otín C et al.: Meta-hallmarks of aging and cancer. Cell Metab 2023. (PMID 36599298) [PubMed] [DOI] Both aging and cancer are characterized by a series of partially overlapping "hallmarks" that we subject here to a meta-analysis. Several hallmarks of aging (i.e., genomic instability, epigenetic alterations, chronic inflammation, and dysbiosis) are very similar to specific cancer hallmarks and hence constitute common "meta-hallmarks," while other features of aging (i.e., telomere attrition and stem cell exhaustion) act likely to suppress oncogenesis and hence can be viewed as preponderantly "antagonistic hallmarks." Disabled macroautophagy and cellular senescence are two hallmarks of aging that exert context-dependent oncosuppressive and pro-tumorigenic effects. Similarly, the equivalence or antagonism between aging-associated deregulated nutrient-sensing and cancer-relevant alterations of cellular metabolism is complex. The agonistic and antagonistic relationship between the processes that drive aging and cancer has bearings for the age-related increase and oldest age-related decrease of cancer morbidity and mortality, as well as for the therapeutic management of malignant disease in the elderly.
