Yeast (Saccharomyces Cerevisiae): Difference between revisions
| (12 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:Saccharomyces cerevisiae SEM.jpg|right|frameless]] | |||
'''''Saccharomyces cerevisiae''''' ('''brewer's yeast''' or '''baker's yeast''') is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like ''[[Escherichia coli]]'' as the model bacterium. ''S. cerevisiae'' cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding.<ref>{{cite book|last=Feldmann|first=Horst|title=Yeast. Molecular and Cell bio|date=2010|publisher=Wiley-Blackwell|isbn=978-3527326099}}</ref> | '''''Saccharomyces cerevisiae''''' ('''brewer's yeast''' or '''baker's yeast''') is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like ''[[Escherichia coli]]'' as the model bacterium. ''S. cerevisiae'' cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding.<ref>{{cite book|last=Feldmann|first=Horst|title=Yeast. Molecular and Cell bio|date=2010|publisher=Wiley-Blackwell|isbn=978-3527326099}}</ref> | ||
==Model Organism== | |||
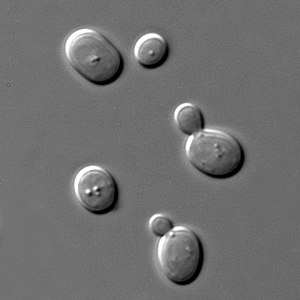
[[File:S cerevisiae under DIC microscopy.jpg|thumb|''S. cerevisiae'', | [[File:S cerevisiae under DIC microscopy.jpg|thumb|''S. cerevisiae'', differential interference contrast image]] | ||
[[Image:20100911 232323 Yeast Live.jpg|thumb|''Saccharomyces cerevisiae''<br />Numbered ticks are 11 micrometers apart.]] | [[Image:20100911 232323 Yeast Live.jpg|thumb|''Saccharomyces cerevisiae''<br />Numbered ticks are 11 micrometers apart.]] | ||
When researchers look for an organism to use in their studies, they look for several traits. Among these are size, generation time, accessibility, manipulation, genetics, conservation of mechanisms, and potential economic benefit. The yeast species '' | When researchers look for an organism to use in their studies, they look for several traits. Among these are size, generation time, accessibility, manipulation, genetics, conservation of mechanisms, and potential economic benefit. The yeast species ''S. pombe'' and ''S. cerevisiae'' are both well studied; these two species diverged approximately 600 to 300 million years ago, and are significant tools in the study of DNA damage and repair mechanisms.<ref>{{cite book |first1= Jac A. |last1=Nickoloff |first2=James E. |last2=Haber |date=2011 |chapter=Mating-Type Control of DNA Repair and Recombination in ''Saccharomyces cerevisiae'' |doi=10.1007/978-1-59259-095-7_5 |pages=107–124 |editor1-first=Jac A. |editor1-last=Nickoloff |editor2-first=Merl F. |editor2-last=Hoekstra |title=DNA Damage and Repair |series=Contemporary Cancer Research |isbn=978-1-59259-095-7|doi-broken-date=1 August 2023 | chapter-url=https://link.springer.com/chapter/10.1007/978-1-59259-095-7_5}}</ref> | ||
''S. cerevisiae'' has developed as a [[model organism]] because it scores favorably on a number of these criteria. | ''S. cerevisiae'' has developed as a [[Model Organism|model organism]] because it scores favorably on a number of these criteria. | ||
* As a single-cell organism, ''S. cerevisiae'' is small with a short generation time (doubling time 1.25–2 hours<ref>{{cite book |editor=Boekhout, T. |editor2=Robert, V. |date=2003 |title=Yeasts in Food: Beneficial and Detrimental aspects |publisher=Behr's Verlag |isbn=978-3-86022-961-3 |page=322 |url=https://books.google.com/books?id=GG-60Vtl81EC |access-date=January 10, 2011}}</ref> at {{convert|30|C|F|disp=or}}) and can be easily | * As a single-cell organism, ''S. cerevisiae'' is small with a short generation time (doubling time 1.25–2 hours<ref>{{cite book |editor=Boekhout, T. |editor2=Robert, V. |date=2003 |title=Yeasts in Food: Beneficial and Detrimental aspects |publisher=Behr's Verlag |isbn=978-3-86022-961-3 |page=322 |url=https://books.google.com/books?id=GG-60Vtl81EC |access-date=January 10, 2011}}</ref> at {{convert|30|C|F|disp=or}}) and can be easily cultured. These are all positive characteristics in that they allow for the swift production and maintenance of multiple specimen lines at low cost. | ||
* ''S. cerevisiae'' divides with meiosis, allowing it to be a candidate for sexual genetics research. | * ''S. cerevisiae'' divides with meiosis, allowing it to be a candidate for sexual genetics research. | ||
* ''S. cerevisiae'' can be | * ''S. cerevisiae'' can be transformed allowing for either the addition of new genes or deletion through homologous recombination. Furthermore, the ability to grow ''S. cerevisiae'' as a haploid simplifies the creation of gene knockout strains. | ||
* As a | * As a eukaryote, ''S. cerevisiae'' shares the complex internal cell structure of plants and animals without the high percentage of non-coding DNA that can confound research in higher eukaryotes. | ||
* ''S. cerevisiae'' research is a strong economic driver, at least initially, as a result of its established use in industry. | * ''S. cerevisiae'' research is a strong economic driver, at least initially, as a result of its established use in industry. | ||
==In the Study of Aging== | |||
For more than five decades ''S. cerevisiae'' has been studied as a model organism to better understand aging and has contributed to the identification of more mammalian genes affecting aging than any other model organism.{{pmid|22768836}} Some of the topics studied using yeast are [[Caloric Restriction|calorie restriction]], as well as in genes and cellular pathways involved in [[Senescent Cells|senescence]]. The two most common methods of measuring aging in yeast are Replicative Life Span (RLS), which measures the number of times a cell divides, and Chronological Life Span (CLS), which measures how long a cell can survive in a non-dividing stasis state.{{pmid|22768836}} Limiting the amount of glucose or amino acids in the growth medium has been shown to increase RLS and CLS in yeast as well as other organisms.{{pmid|17530929}} At first, this was thought to increase RLS by up-regulating the [[SIRT2|sir2]] enzyme, however it was later discovered that this effect is independent of sir2. Over-expression of the genes sir2 and fob1 has been shown to increase RLS by preventing the accumulation of extrachromosomal rDNA circles, which are thought to be one of the causes of senescence in yeast.{{pmid|17530929}} The effects of dietary restriction may be the result of a decreased signaling in the TOR cellular pathway.{{pmid|22768836}} This pathway modulates the cell's response to nutrients, and mutations that decrease TOR activity were found to increase CLS and RLS.{{pmid|22768836}}{{pmid|17530929}} This has also been shown to be the case in other animals.{{pmid|22768836}}{{pmid|17530929}} A yeast mutant lacking the genes Sch9 and Ras2 has recently been shown to have a tenfold increase in chronological lifespan under conditions of calorie restriction and is the largest increase achieved in any organism.{{pmid|18225956}} | |||
Mother cells give rise to progeny buds by mitotic divisions, but undergo replicative aging over successive generations and ultimately die. However, when a mother cell undergoes meiosis and gametogenesis, lifespan is reset.{{pmid|21700873}} The replicative potential of gametes (spores) formed by aged cells is the same as gametes formed by young cells, indicating that age-associated damage is removed by meiosis from aged mother cells. This observation suggests that during meiosis removal of age-associated damages leads to rejuvenation. However, the nature of these damages remains to be established. | |||
During starvation of non-replicating ''S. cerevisiae'' cells, reactive oxygen species increase leading to the accumulation of DNA damages such as apurinic/apyrimidinic sites and double-strand breaks.{{pmid|20223252}} Also in non-replicating cells the ability to repair endogenous double-strand breaks declines during chronological aging.{{pmid|30410502}} | |||
== See Also == | |||
* [[Model Organism]] | |||
* {{SeeWikipedia|Saccharomyces cerevisiae}} | |||
== References == | == References == | ||
<references /> | <references /> | ||
[[Category:Model Organism]] | |||
Latest revision as of 03:46, 22 December 2023
Saccharomyces cerevisiae (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been originally isolated from the skin of grapes. It is one of the most intensively studied eukaryotic model organisms in molecular and cell biology, much like Escherichia coli as the model bacterium. S. cerevisiae cells are round to ovoid, 5–10 μm in diameter. It reproduces by budding.[1]
Model Organism
When researchers look for an organism to use in their studies, they look for several traits. Among these are size, generation time, accessibility, manipulation, genetics, conservation of mechanisms, and potential economic benefit. The yeast species S. pombe and S. cerevisiae are both well studied; these two species diverged approximately 600 to 300 million years ago, and are significant tools in the study of DNA damage and repair mechanisms.[2]
S. cerevisiae has developed as a model organism because it scores favorably on a number of these criteria.
- As a single-cell organism, S. cerevisiae is small with a short generation time (doubling time 1.25–2 hours[3] at 30 °C or 86 °F) and can be easily cultured. These are all positive characteristics in that they allow for the swift production and maintenance of multiple specimen lines at low cost.
- S. cerevisiae divides with meiosis, allowing it to be a candidate for sexual genetics research.
- S. cerevisiae can be transformed allowing for either the addition of new genes or deletion through homologous recombination. Furthermore, the ability to grow S. cerevisiae as a haploid simplifies the creation of gene knockout strains.
- As a eukaryote, S. cerevisiae shares the complex internal cell structure of plants and animals without the high percentage of non-coding DNA that can confound research in higher eukaryotes.
- S. cerevisiae research is a strong economic driver, at least initially, as a result of its established use in industry.
In the Study of Aging
For more than five decades S. cerevisiae has been studied as a model organism to better understand aging and has contributed to the identification of more mammalian genes affecting aging than any other model organism.[4] Some of the topics studied using yeast are calorie restriction, as well as in genes and cellular pathways involved in senescence. The two most common methods of measuring aging in yeast are Replicative Life Span (RLS), which measures the number of times a cell divides, and Chronological Life Span (CLS), which measures how long a cell can survive in a non-dividing stasis state.[4] Limiting the amount of glucose or amino acids in the growth medium has been shown to increase RLS and CLS in yeast as well as other organisms.[5] At first, this was thought to increase RLS by up-regulating the sir2 enzyme, however it was later discovered that this effect is independent of sir2. Over-expression of the genes sir2 and fob1 has been shown to increase RLS by preventing the accumulation of extrachromosomal rDNA circles, which are thought to be one of the causes of senescence in yeast.[5] The effects of dietary restriction may be the result of a decreased signaling in the TOR cellular pathway.[4] This pathway modulates the cell's response to nutrients, and mutations that decrease TOR activity were found to increase CLS and RLS.[4][5] This has also been shown to be the case in other animals.[4][5] A yeast mutant lacking the genes Sch9 and Ras2 has recently been shown to have a tenfold increase in chronological lifespan under conditions of calorie restriction and is the largest increase achieved in any organism.[6]
Mother cells give rise to progeny buds by mitotic divisions, but undergo replicative aging over successive generations and ultimately die. However, when a mother cell undergoes meiosis and gametogenesis, lifespan is reset.[7] The replicative potential of gametes (spores) formed by aged cells is the same as gametes formed by young cells, indicating that age-associated damage is removed by meiosis from aged mother cells. This observation suggests that during meiosis removal of age-associated damages leads to rejuvenation. However, the nature of these damages remains to be established.
During starvation of non-replicating S. cerevisiae cells, reactive oxygen species increase leading to the accumulation of DNA damages such as apurinic/apyrimidinic sites and double-strand breaks.[8] Also in non-replicating cells the ability to repair endogenous double-strand breaks declines during chronological aging.[9]
See Also
- Model Organism
- Wikipedia - Saccharomyces cerevisiae
References
- ↑ Feldmann; "Yeast. Molecular and Cell bio" , ISBN: 978-3527326099
- ↑ Nickoloff et al.; "DNA Damage and Repair" , ISBN: 978-1-59259-095-7
- ↑ "Yeasts in Food: Beneficial and Detrimental aspects" , pp. 322 , ISBN: 978-3-86022-961-3
- ↑ 4.0 4.1 4.2 4.3 4.4 Longo VD et al.: Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab 2012. (PMID 22768836) [PubMed] [DOI] [Full text] Saccharomyces cerevisiae has directly or indirectly contributed to the identification of arguably more mammalian genes that affect aging than any other model organism. Aging in yeast is assayed primarily by measurement of replicative or chronological life span. Here, we review the genes and mechanisms implicated in these two aging model systems and key remaining issues that need to be addressed for their optimization. Because of its well-characterized genome that is remarkably amenable to genetic manipulation and high-throughput screening procedures, S. cerevisiae will continue to serve as a leading model organism for studying pathways relevant to human aging and disease.
- ↑ 5.0 5.1 5.2 5.3 Kaeberlein M et al.: Recent developments in yeast aging. PLoS Genet 2007. (PMID 17530929) [PubMed] [DOI] [Full text] In the last decade, research into the molecular determinants of aging has progressed rapidly and much of this progress can be attributed to studies in invertebrate eukaryotic model organisms. Of these, single-celled yeast is the least complicated and most amenable to genetic and molecular manipulations. Supporting the use of this organism for aging research, increasing evidence has accumulated that a subset of pathways influencing longevity in yeast are conserved in other eukaryotes, including mammals. Here we briefly outline aging in yeast and describe recent findings that continue to keep this "simple" eukaryote at the forefront of aging research.
- ↑ Wei M et al.: Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 2008. (PMID 18225956) [PubMed] [DOI] [Full text] Calorie restriction (CR), the only non-genetic intervention known to slow aging and extend life span in organisms ranging from yeast to mice, has been linked to the down-regulation of Tor, Akt, and Ras signaling. In this study, we demonstrate that the serine/threonine kinase Rim15 is required for yeast chronological life span extension caused by deficiencies in Ras2, Tor1, and Sch9, and by calorie restriction. Deletion of stress resistance transcription factors Gis1 and Msn2/4, which are positively regulated by Rim15, also caused a major although not complete reversion of the effect of calorie restriction on life span. The deletion of both RAS2 and the Akt and S6 kinase homolog SCH9 in combination with calorie restriction caused a remarkable 10-fold life span extension, which, surprisingly, was only partially reversed by the lack of Rim15. These results indicate that the Ras/cAMP/PKA/Rim15/Msn2/4 and the Tor/Sch9/Rim15/Gis1 pathways are major mediators of the calorie restriction-dependent stress resistance and life span extension, although additional mediators are involved. Notably, the anti-aging effect caused by the inactivation of both pathways is much more potent than that caused by CR.
- ↑ Unal E et al.: Gametogenesis eliminates age-induced cellular damage and resets life span in yeast. Science 2011. (PMID 21700873) [PubMed] [DOI] [Full text] Eukaryotic organisms age, yet detrimental age-associated traits are not passed on to progeny. How life span is reset from one generation to the next is not known. We show that in budding yeast resetting of life span occurs during gametogenesis. Gametes (spores) generated by aged cells show the same replicative potential as gametes generated by young cells. Age-associated damage is no longer detectable in mature gametes. Furthermore, transient induction of a transcription factor essential for later stages of gametogenesis extends the replicative life span of aged cells. Our results indicate that gamete formation brings about rejuvenation by eliminating age-induced cellular damage.
- ↑ Steinboeck F et al.: The relevance of oxidative stress and cytotoxic DNA lesions for spontaneous mutagenesis in non-replicating yeast cells. Mutat Res 2010. (PMID 20223252) [PubMed] [DOI] Mutations arising during times of cell cycle-arrest may considerably contribute to aging and cancerogenesis. Endogenous oxidative stress could be one of the major triggers for these mutations. We used Saccharomyces cerevisiae cells, arrested by starvation for the essential amino acid lysine, to study the occurrence of reactive oxygen species (ROS), abasic (AP) sites and double strand breaks (DSBs). Furthermore, we analyzed the mutation frequencies in resting wild type cells and in cells deficient for Apn1 (with an impaired base excision repair) or Dnl4 (with an inactivated non-homologous end joining (NHEJ) DSB repair pathway) by monitoring reversions of an auxotrophy-causing frameshift in the LYS2 gene. By fluorescence methods, we observed a distinct increase of ROS-affected cells in the course of starvation-induced cell cycle-arrest. In addition, we could reveal that AP sites and DSBs accumulated under these conditions. The frequency of spontaneous frameshift mutations in wild type cells was decreased to 50% upon addition of 6mM N-acetyl cysteine. However, this radical scavenger had no effect in Dnl4-deficient cells. Our results support the hypothesis that (via an active NHEJ DSB repair pathway) the incidence of spontaneous frameshift mutations in a cell cycle-arrested state is considerably governed by oxidative stress.
- ↑ Pongpanich M et al.: Pathologic Replication-Independent Endogenous DNA Double-Strand Breaks Repair Defect in Chronological Aging Yeast. Front Genet 2018. (PMID 30410502) [PubMed] [DOI] [Full text] Reduction of physiologic replication-independent endogenous DNA double strand breaks (Phy-RIND-EDSBs) in chronological aging yeast increases pathologic RIND-EDSBs (Path-RIND-EDSBs). Path-RIND-EDSBs can occur spontaneously in non-dividing cells without any inductive agents, and they must be repaired immediately otherwise their accumulation can lead to senescence. If yeasts have DSB repair defect, retention of Path-RIND-EDSBs can be found. Previously, we found that Path-RIND-EDSBs are not only produced but also retained in chronological aging yeast. Here, we evaluated if chronological aging yeasts have a DSB repair defect. We found a significant accumulation of Path-RIND-EDSBs around the same level in aging cells and caffeine treated cells and at a much higher level in the DSB repair mutant cells. Especially in the mutant, some unknown sequence was found inserted at the breaks. In addition, % difference of cell viability between HO induced and non-induced cells was significantly greater in aging cells. Our results suggested that RIND-EDSBs repair efficiency declines, but is not absent, in chronological aging yeast which might promote senescence phenotype. When a repair protein is deficient, an alternative pathway might be employed or an end modification process might occur as inserted sequences at the breaks were observed. Restoring repair defects might slow down the deterioration of cells from chronological aging.