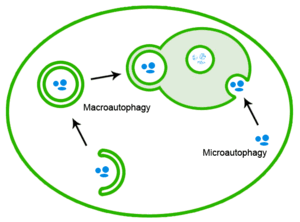
Disabled Macroautophagy
Disabled macroautophagy is a hallmark of aging and refers to the impairment or inefficiency of the cellular process known as macroautophagy. Macroautophagy is a critical aspect of the cell's recycling system, responsible for the degradation and recycling of damaged organelles, misfolded proteins, and other cellular debris. It involves the formation of autophagosomes, which encapsulate the cellular waste and then fuse with lysosomes to degrade the contents. This process is vital for maintaining cellular health and function, particularly under stress conditions or during aging.
Importance of Macroautophagy
Macroautophagy is essential for cell survival, differentiation, development, and homeostasis. It is particularly significant in response to nutrient starvation, as it provides an internal source of nutrients by recycling cellular components. Moreover, by clearing damaged and potentially toxic materials, macroautophagy contributes to cellular quality control and prevents the accumulation of cellular damage that can lead to diseases such as cancer, neurodegeneration, and infections.
Consequences of Disabled Macroautophagy
When macroautophagy is disabled or impaired:
- Accumulation of Damaged Components: Cells accumulate damaged proteins and organelles, which can interfere with normal cellular functions and lead to cellular aging and death.
- Increased Oxidative Stress: Impaired clearance of damaged mitochondria leads to increased production of reactive oxygen species (ROS), contributing to oxidative stress and further damaging cellular components.
- Disease Progression: Disabled macroautophagy has been implicated in the progression of various diseases, including neurodegenerative diseases (like Alzheimer's and Parkinson's), cancer, and metabolic disorders.
- Aging: Impaired autophagy is associated with many aging-related phenotypes and may contribute to the aging process itself by allowing the accumulation of cellular damage over time.
Therapeutic Implications
Understanding and targeting the pathways involved in macroautophagy has significant therapeutic potential. Enhancing or restoring macroautophagy could help clear protein aggregates and damaged organelles, potentially slowing the progression of neurodegenerative diseases, extending lifespan, and improving overall health. Various strategies are being explored, including pharmacological agents that can induce or inhibit specific steps of the autophagy process.
See Also
- Hallmarks of Aging
- Wikipedia - Macroautophagy
Further Reading
References
- ↑ Hansen M et al.: Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 2018. (PMID 30006559) [PubMed] [DOI] [Full text] Autophagy is a conserved process that catabolizes intracellular components to maintain energy homeostasis and to protect cells against stress. Autophagy has crucial roles during development and disease, and evidence accumulated over the past decade indicates that autophagy also has a direct role in modulating ageing. In particular, elegant studies using yeasts, worms, flies and mice have demonstrated a broad requirement for autophagy-related genes in the lifespan extension observed in a number of conserved longevity paradigms. Moreover, several new and interesting concepts relevant to autophagy and its role in modulating longevity have emerged. First, select tissues may require or benefit from autophagy activation in longevity paradigms, as tissue-specific overexpression of single autophagy genes is sufficient to extend lifespan. Second, selective types of autophagy may be crucial for longevity by specifically targeting dysfunctional cellular components and preventing their accumulation. And third, autophagy can influence organismal health and ageing even non-cell autonomously, and thus, autophagy stimulation in select tissues can have beneficial, systemic effects on lifespan. Understanding these mechanisms will be important for the development of approaches to improve human healthspan that are based on the modulation of autophagy.
- ↑ Wong SQ et al.: Autophagy in aging and longevity. Hum Genet 2020. (PMID 31144030) [PubMed] [DOI] [Full text] Our understanding of the process of autophagy and its role in health and diseases has grown remarkably in the last two decades. Early work established autophagy as a general bulk recycling process which involves the sequestration and transport of intracellular material to the lysosome for degradation. Currently, autophagy is viewed as a nexus of metabolic and proteostatic signalling that can determine key physiological decisions from cell fate to organismal lifespan. Here, we review the latest literature on the role of autophagy and lysosomes in stress response and longevity. We highlight the connections between autophagy and metabolic processes, the network associated with its regulation, and the links between autophagic dysfunction, neurodegenerative diseases, and aging.