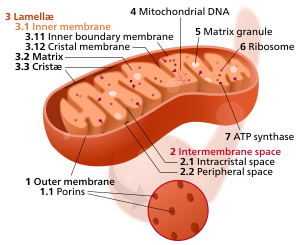
Mitochondrial Dysfunction
Mitochondrial Dysfunction is recognized as a central hallmark of aging and contributes to the pathogenesis of a wide range of age-related diseases. Mitochondria are known as the "powerhouses of the cell," responsible for producing the majority of the cellular energy in the form of adenosine triphosphate (ATP) through a process called oxidative phosphorylation. Beyond energy production, mitochondria are also involved in a variety of other important processes, including calcium homeostasis, apoptotic cell death, and the generation of reactive oxygen species (ROS). As organisms age, mitochondrial function tends to decline, leading to reduced energy production, increased oxidative damage, and impaired cellular function.
The Role of Mitochondria in Cellular Function
Mitochondria play several vital roles in maintaining cellular and organismal health:
- Energy Production: Through oxidative phosphorylation, mitochondria convert nutrients into ATP, the main energy currency of the cell.
- Regulation of Metabolic Pathways: Mitochondria are involved in the regulation of various metabolic pathways, including the citric acid cycle, fatty acid oxidation, and amino acid metabolism.
- Apoptosis: Mitochondria release factors that are crucial in the intrinsic pathway of apoptosis, or programmed cell death.
- Calcium Homeostasis: Mitochondria can sequester and release calcium ions, thus contributing to cellular calcium signaling and homeostasis.
- Reactive Oxygen Species (ROS) Production: Mitochondria are a major source of ROS, which are byproducts of oxidative phosphorylation. While ROS can act as signaling molecules, excessive ROS lead to oxidative damage and cellular stress.
Mechanisms of Mitochondrial Dysfunction
Mitochondrial dysfunction can arise from various sources and mechanisms:
- Genetic Mutations: Mutations in both mitochondrial DNA (mtDNA) and nuclear DNA (nDNA) that encode mitochondrial components can lead to impaired function.
- Accumulation of Damaged mtDNA: Mitochondria have their own DNA, and damage to mtDNA can accumulate over time, leading to a decline in mitochondrial function.
- Impaired Mitophagy: Mitophagy is the selective degradation of mitochondria by autophagy. Dysfunction in this process leads to the accumulation of damaged mitochondria.
- Alterations in Fission and Fusion: Mitochondria constantly undergo fission (division) and fusion (joining), processes that are essential for maintaining mitochondrial quality and function. Disruption in these processes can lead to dysfunction.
- Oxidative Stress: Excessive production of ROS can damage proteins, lipids, and DNA, leading to impaired mitochondrial function and signaling.
Implications of Mitochondrial Dysfunction
Mitochondrial dysfunction has widespread implications for cellular health and organismal aging:
- Energetic Decline: As mitochondrial function deteriorates, cells experience an energy deficit, affecting various cellular processes and leading to a decline in tissue function.
- Increased Oxidative Damage: Mitochondrial dysfunction often leads to increased ROS production and oxidative stress, contributing to further mitochondrial damage and cellular aging.
- Apoptotic Signaling: Dysfunction in mitochondria can trigger apoptotic pathways, leading to cell death and tissue degeneration.
- Inflammation: Mitochondrial dysfunction can activate inflammatory pathways, contributing to chronic inflammation associated with many age-related diseases.
- Age-related Diseases: Mitochondrial dysfunction is implicated in a host of age-related diseases, including neurodegenerative diseases, cardiovascular diseases, diabetes, and cancer.
Therapeutic Interventions and Research
Research into mitochondrial dysfunction has led to the exploration of various strategies to preserve or restore mitochondrial function:
- Antioxidants: Compounds that can neutralize ROS are being investigated for their potential to reduce oxidative damage and improve mitochondrial function.
- Mitochondrial Biogenesis Stimulators: Drugs or interventions that can stimulate the production of new mitochondria are of interest for reversing the age-related decline in mitochondrial content.
- Gene Therapy: Approaches to repair or replace defective mitochondrial genes are under exploration.
- Diet and Lifestyle: Dietary interventions, caloric restriction, and exercise have been shown to influence mitochondrial health and function.