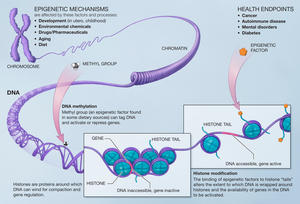
Epigenetic Alterations
Epigenetic alterations is one of the identified hallmarks of aging. These aging-associated epigenetic changes include DNA methylation, histone modification, chromatin remodeling, non-coding RNA (ncRNA) regulation, and RNA modification, all of which participate in the regulation of the aging process, and hence contribute to aging-related diseases.
Epigenetic modifications play a crucial role in the aging process by affecting gene expression without altering the DNA sequence itself. These modifications are influenced by a range of factors including environmental influences, lifestyle choices, and age-related physiological changes.
Primary Mechanisms of Epigenetic Alterations
- DNA Methylation: The addition of methyl groups to the DNA molecule, particularly at CpG sites, leading to changes in gene expression. This process is integral to the aging process, with patterns of hypomethylation and hypermethylation being observed in different genomic regions as one ages.
- Histone Modifications: Histones, around which DNA is wound, can undergo various chemical modifications such as methylation and acetylation. These changes can affect the accessibility of DNA to transcription factors and, consequently, gene expression.
- Chromatin Remodeling: The structure of chromatin changes over time, influencing the compactness of DNA and its accessibility for transcription. This remodeling is a dynamic process and plays a key role in aging.
- Non-Coding RNA Regulation: ncRNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), can regulate gene expression at various levels. These molecules are key players in the aging process, influencing numerous age-related pathways.
- RNA Modification: Post-transcriptional modifications of RNA molecules also contribute to the aging process by altering the stability, localization, and translation of RNA.
Impact on Longevity and Age-Related Diseases
Epigenetic alterations can lead to a range of age-related changes and diseases:
- Cellular Senescence: Alterations in epigenetic regulation can contribute to the onset of cellular senescence, a state where cells no longer divide and accumulate with age.
- Chronic Diseases: Many age-related diseases, such as certain cancers, cardiovascular diseases, and neurodegenerative disorders, have been linked to epigenetic changes.
- Biological Aging: Epigenetic clocks, which assess biological age based on DNA methylation patterns, provide insights into an individual’s biological aging process, which may differ from their chronological age.
Research and Intervention Strategies
Ongoing research is focused on understanding the precise role of epigenetic alterations in aging and longevity:
- Diet and Lifestyle: Factors such as diet, exercise, and environmental exposures can significantly influence epigenetic patterns and potentially mitigate aging processes.
- Pharmacological Approaches: Targeting epigenetic modifications with drugs, like histone deacetylase inhibitors, is a promising strategy for modulating aging and treating age-related diseases.
- Gene Editing and Epigenetic Therapy: Advanced techniques like CRISPR/Cas9 provide opportunities for targeted epigenetic modifications, offering potential pathways for anti-aging interventions.
Conclusion
Epigenetic alterations are a fundamental aspect of the aging process and represent a key hallmark of aging. These modifications offer a complex but insightful view into the biological mechanisms underlying aging and age-related diseases. By furthering our understanding of epigenetic mechanisms and developing interventions that can modulate these changes, there is potential for significant advancements in promoting healthspan and potentially delaying the onset of age-related diseases. Continued research in this area holds promise for novel therapeutic approaches and a deeper understanding of the aging process.
Further Reading
- 2022, Epigenetic regulation of aging: implications for interventions of aging and diseases [1]
See Also
- Hallmarks of Aging
- Epigenetic Clocks
- Wikipedia - Epigenetics
References
- ↑ Wang K et al.: Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduct Target Ther 2022. (PMID 36336680) [PubMed] [DOI] [Full text] Aging is accompanied by the decline of organismal functions and a series of prominent hallmarks, including genetic and epigenetic alterations. These aging-associated epigenetic changes include DNA methylation, histone modification, chromatin remodeling, non-coding RNA (ncRNA) regulation, and RNA modification, all of which participate in the regulation of the aging process, and hence contribute to aging-related diseases. Therefore, understanding the epigenetic mechanisms in aging will provide new avenues to develop strategies to delay aging. Indeed, aging interventions based on manipulating epigenetic mechanisms have led to the alleviation of aging or the extension of the lifespan in animal models. Small molecule-based therapies and reprogramming strategies that enable epigenetic rejuvenation have been developed for ameliorating or reversing aging-related conditions. In addition, adopting health-promoting activities, such as caloric restriction, exercise, and calibrating circadian rhythm, has been demonstrated to delay aging. Furthermore, various clinical trials for aging intervention are ongoing, providing more evidence of the safety and efficacy of these therapies. Here, we review recent work on the epigenetic regulation of aging and outline the advances in intervention strategies for aging and age-associated diseases. A better understanding of the critical roles of epigenetics in the aging process will lead to more clinical advances in the prevention of human aging and therapy of aging-related diseases.